NUCLEIC ACID BASES
Tautomerism of nucleic acid bases
Much effort has been devoted to the identification of preferred tautomers of nucleobases since the structure of nucleic acids and its base pairs were first reported. The best experimental approach to address the structural preferences of nucleobases is to place them under isolation conditions in the gas phase, cooled in a supersonic expansion. Under these conditions, the various tautomers/conformers can coexist and are not affected by the bulk effects of their native environments, which normally mask their intrinsic molecular properties. The main restriction to the gas-phase study of these building blocks is the difficulty in their vaporization owing to their high melting points (ranging from 316 ºC for guanine to 365 ºC for adenine) and associated low vapor pressures.
The success of LA-MB-FTMW experiments to the study of coded amino acids prompted their application to nucleic acids uracil, thymine, guanine, and cytosine, as well as the monohydrates of uracil and thymine. This technique gives a precise interpretation of the structure and relative energies of the different forms of nucleic acid bases to solve apparent discrepancies between the previous studies. The rotational constants, which are the main tool to identify the different forms of a biomolecule, had a minor role when trying to discern between the different tautomers of a nucleobase. It is the quadrupole coupling hyperfine structure caused by the presence of 14N nuclei which constitute authentically the fingerprints of every tautomer.
Featured work
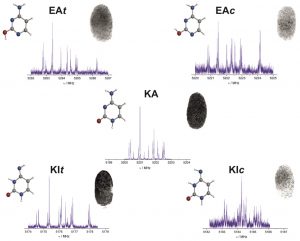
All Five Forms of Cytosine Revealed in the Gas Phase,
Angew. Chem. 2013, 125, 2387-2390
Give me five! All five tautomers and conformers of cytosine were characterized in the gas phase by laser ablation molecular beam Fourier transform microwave spectroscopy. The spectra were assigned unambiguously on the basis of the hyperfine structure due to the three 14N nuclei (see picture; N blue, O red). The relative energies of the identified species were estimated from the relative intensities of the spectra.
Last contributions
*Accurate molecular structure and spectroscopic properties of nucleobases: a combined
computational–microwave investigation of 2-thiouracil as a case study,
Phys. Chem. Chem. Phys., 2013, 15, 16965-16975
*Rotational spectral signatures of four tautomers of guanine,
Angew. Chem. Int. Ed., 2009, 48, 6141-6143
*Probing thymine with Laser Ablation Molecular Beam Fourier Transform microwave spectroscopy,
J. Chem. Phys., 2007, 126, 191103
*The structure of uracil: A Laser Ablation rotational study,
J. Phys. Chem. A, 2007, 111, 3443-3445