Structure and conformational behavior of monosaccharides
Carbohydrates are one of the most versatile biochemical building blocks, widely acting in energetic, structural, or recognition processes. The importance of its structure has been the driving force behind the development of methods for elucidating the shape of their building blocks, monosaccharides. Thus, it comes as no surprise that 3D structures and relative stability of conformers of monosaccharides continue to be an area of great research interest. The subtle variation in hydroxyl arrangement is thought to account for differences in chemical and physical properties of the sugars. This is also relevant to distinguish between different conformers. Additionally, monosaccharides are also of interest in the field of astrophysics. The availability of rotational data has been the main bottleneck for examining the presence of these building blocks in the interstellar medium (ISM). Based on the rotational spectra identification, the simplest C2 sugar of glycolaldehyde has been identified, but has yet to detect the C3 sugar of glyceraldehyde. The experimental results obtained in condensed phases seem to indicate that a subtle balance between intrinsic and environmental effects governs the conformational preferences of monosaccharides;
subtle balance between intrinsic and environmental effects governs the conformational preferences of monosaccharides; the structure and relative stability of isolated sugars are different from their counterparts in solution. To separate these contributions, it is crucial to obtain data on the isolated monosaccharides in the gas phase. This highlights the importance of generating sugars in isolated conditions, free from the influence of environmental effects to determine its intrinsic conformational properties relevant to understand its biological activity. Fourier transform microwave spectroscopy techniques in supersonic jets, combined with laser ablation techniques, can bring intact monosaccharides into the gas phase for structural investigation. The low-temperature environment of a supersonic expansion provides the ideal medium for preparing individual conformers of sugars in virtual isolation conditions, ready to be interrogated by a short burst of microwave radiation. To date, rotational investigations of monosaccharides have been carried out for C4 sugars, C5 sugars, and C6sugars.
Featured results
The conformational behaviour of free D-glucose-at last,
Chem. Sci., 2014, 5,515-522
The conformational behaviour of isolated D-glucose has been revealed in this work using Fourier transform microwave spectroscopy coupled with laser ablation of crystalline α- and β-glucopyranose samples. Four conformers of α-D-glucopyranose and three of β-D-glucopyranose have been unequivocally identified on the basis of the spectroscopic rotational parameters in conjunction with ab initio predictions. Stereoelectronic hyperconjugative factors, like those associated with anomeric or gauche effects, as well as the cooperative OH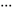 O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.
O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.
α-D Glucose
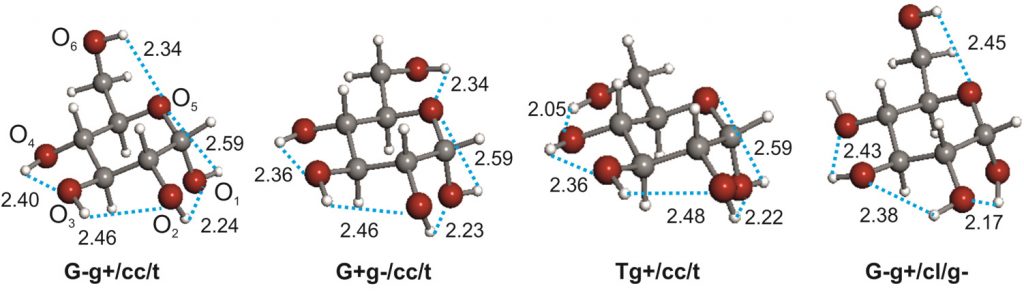
β-D Glucose
Last contributions
*The shape of D-glucosamine,
Phys. Chem. Chem. Phys., 2014, 16, 23244-23250
*Erythrose revealed as furanose forms,
Chem.Commun., 2013, 49, 10826-10828
*Six Pyranoside Forms of Free 2-Deoxy-D-ribose,
Angew. Chem. Int. Ed., 2013, 52, 11840-11845
 O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.
O chains extended along the entire molecule, are the main factors driving the conformational behaviour. The most abundant conformers exhibit a counter-clockwise arrangement (cc) of the network of intramolecular hydrogen bonds.


