Who Natural amino acids and dipeptides
The conformational behaviour of amino acids is of critical interest to understand the dynamical role of these molecules in protein or polypeptide formation. Consequently, extensive structural research has been conducted on amino acids in their natural solid-state phase. However, in the solid state amino acids present a zwitterionic structure (i.e. a bipolar, ionized form of the type +H3N-CH(R)-COO-), which does not occur in the polypeptide chain. In peptides and proteins amino acids participate in their neutral forms. To obtain the structure of the neutral amino acids research should be conducted in the gas phase, in an atmosphere that is essentially free of intermolecular interactions with other partners. In particular, the collisionless environment of a supersonic jet seems particularly well suited for such studies. Rotational spectroscopy is the only technique that, thanks to its inherently superior resolution, can distinguish unambiguously between different isomers, conformers, or isotopomers and provide accurate structural information directly comparable to the in vacuo theoretical predictions.
However, amino acids are difficult to vaporize due to their high melting points and thermal unstability. The analysis of the rotational spectrum of natural amino acids started in the late 1970s on glycine and alanine, but it was refrained for a long time by the difficulties to bring these compounds into gas phase. We have recently impulsed this field with the combination of laser ablation with Fourier transform microwave spectroscopy in supersonic jets. Over the last years, we have studied the gas-phase conformational behaviour and structure of 18 natural amino acids (histidine, glutamic acid, asparagine,…) and non natural (AC3C, BAIBA, …), to understand the role of intramolecular hydrogen bond interactions in the stabilization of the preferred conformations.
Tautomerism in Neutral Histidine, Angew.
Chem. Int. Ed. 2014, 53, 11015-11018
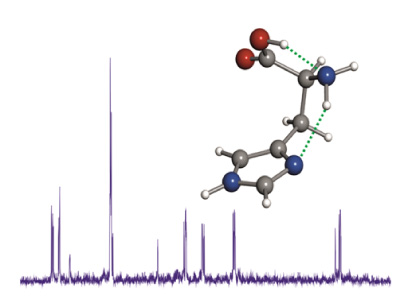
Histidine is an important natural amino acid, involved in many relevant biological processes, which, because of its physical properties, proved difficult to characterize experimentally in its neutral form. In this work, neutral histidine has been generated in the gas phase by laser ablation of solid samples and its NεH tautomeric form unraveled through its rotational spectrum. The quadrupole hyperfine structure, arising from the existing three 14N nuclei, constituted a site-specifically probe for revealing the tautomeric form as well as the side chain configuration of this proteogenic amino acid.
Last contributions
*The conformational locking of asparagine,
Chem. Commun. 2012, 48, 5934
*Preferred Conformers of Proteinogenic Glutamic Acid, J. Am.
Chem. Soc. 2012, 134, 2305-2312